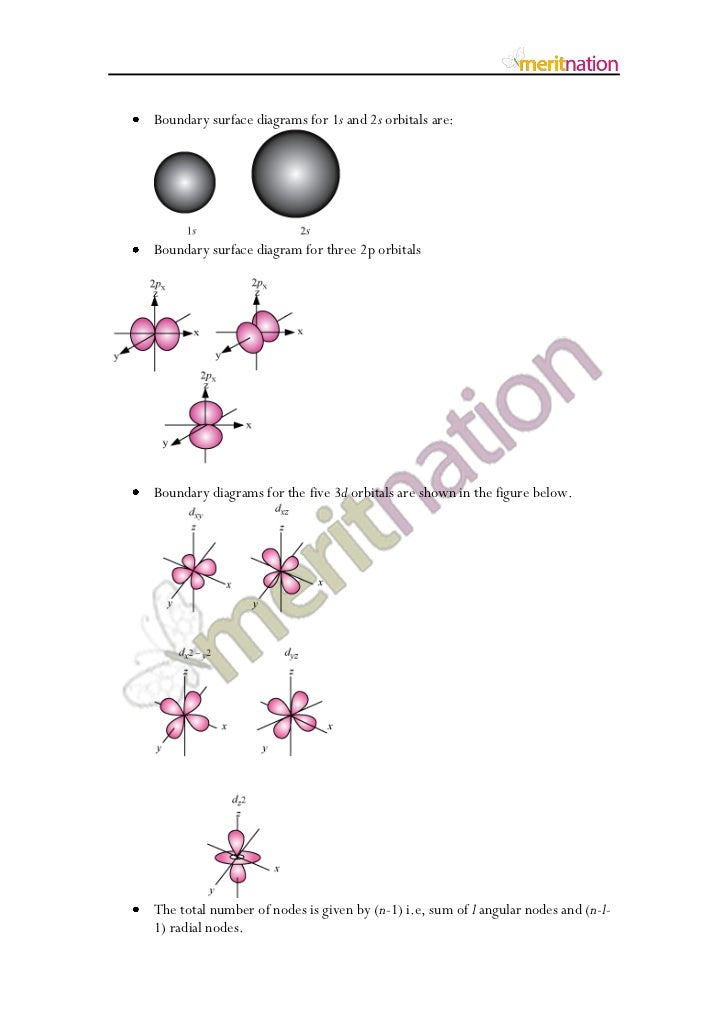
Boundary Surface 5s Orbital
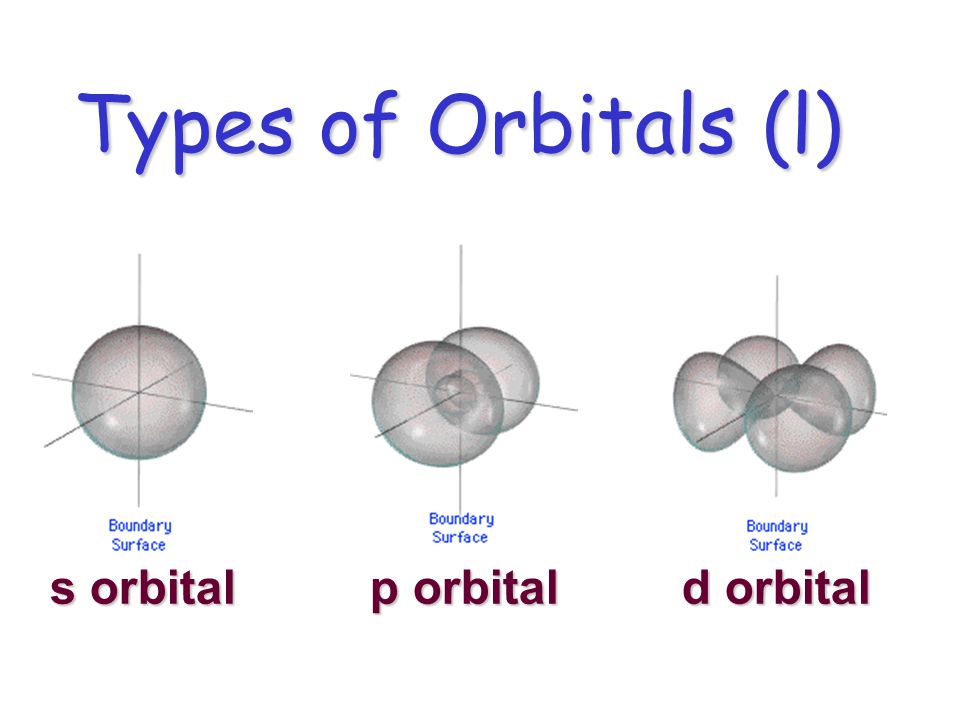
The boundary surface diagram of s orbital is spherical, so it will be spherical for 1s, 2s, 3s and 4s or for any general ns.

Boundary surface 5s orbital. We have undertaken a simplified calculation of orbital forcing back through the Cretaceous to the Late to Middle Jurassic from 65 to 190 Ma. M l =0, ±1, ±2,. Sketch the p x and d xz orbitals.
The 2s orbital is shown below, once again represented by a dot density diagram, a boundary surface diagram, and a rotating image. In the above dot- population picture, the relative probability. In the molecular orbital theory of H 2, we consider the molecular orbitals as made up of the symmetric and antisymmetric combination of the individual 1s atomic orbitals on the two atoms.In general, however, there is more than one occupied orbital in the original atoms.
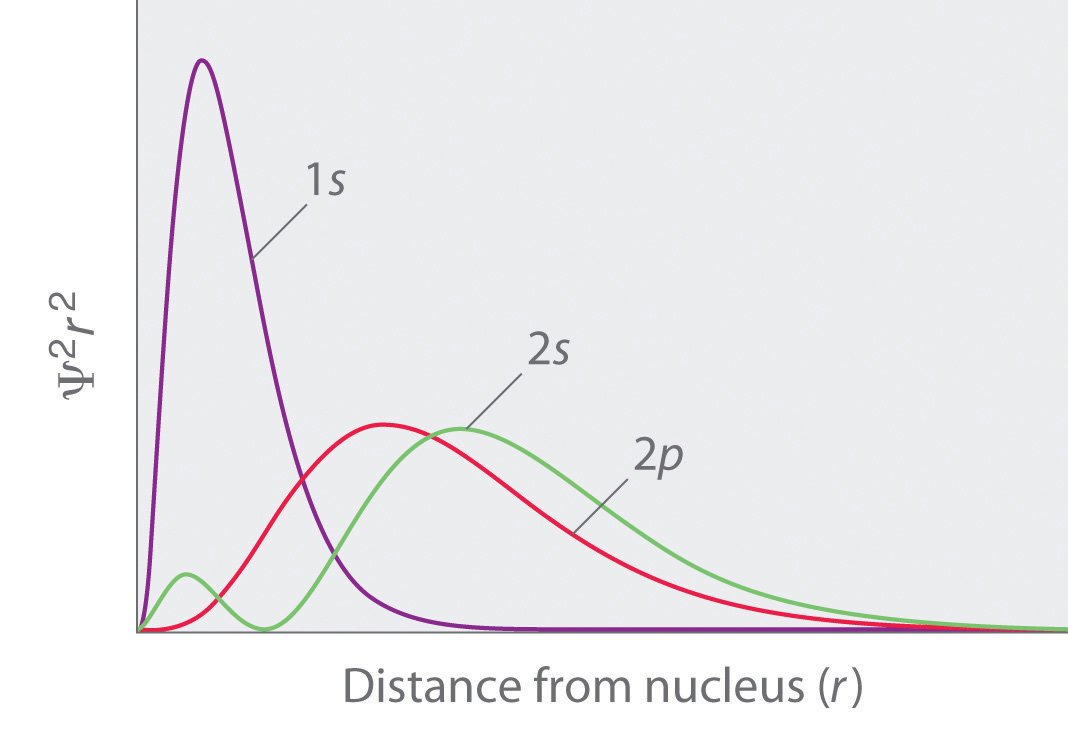
Note the presence of the radial node in 3p z orbital. This means that the probability of finding the electron in s-orbital is the same in all directions at a particular distance. For example, d xy orbital has two nodal planes passing through the origin and bisecting the principal axes.
An orbital is the quantum mechanical refinement of Bohr’s orbit. Generally speaking, the number n determines the size and energy of the orbital:. The transition metal series is defined by the progressive filling of the 3d orbitals.These five orbitals have the following m l values:.
Shapes of atomic orbitals:. Be sure to show and label the coordinates. An orbital is a region in space where the probability of finding and electron is greatest.
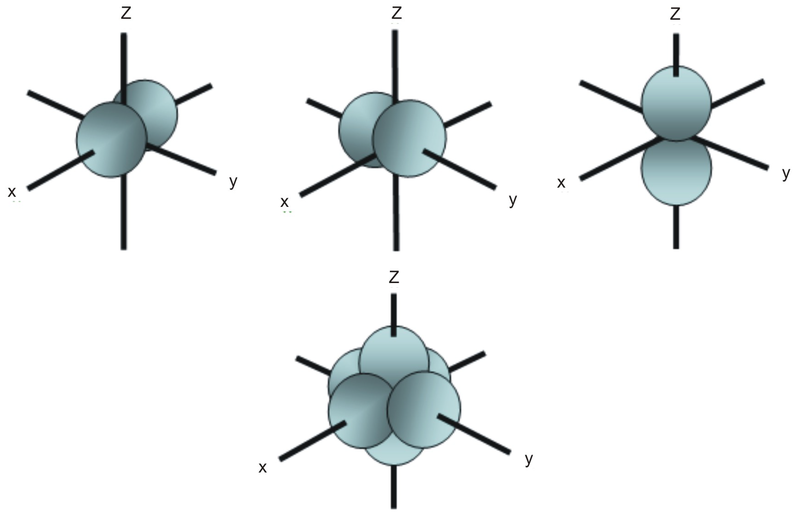
Boundary surface diagrams of constant probability density for different orbitals give a fairly good representation of the shapes of the orbitals. Learn this topic by watching Orbital Shapes Concept Videos. The boundary surface of a p orbital therefore consists of two lobes projecting from the nucleus.
The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. That is, the size of the orbital increases as n increases. The boundary surfaces of the p orbitals are shown in Figure 3.
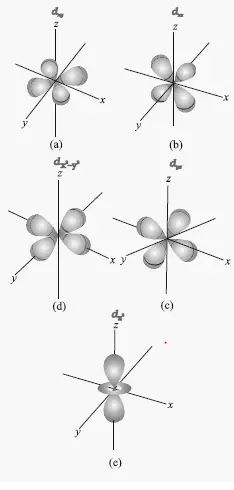
Shapes of d orbital. Draw the shapes (boundary surfaces) of the following orbitals 2py 3dz2 3dx2 y2 - Chemistry - Structure of Atom. Be sure to show and label the axes.
This overlap leads to the formation of a bonding molecular orbital with two nodal planes which contain the internuclear axis and go through both atoms. The cross term 2 ´ ls A ´ ls B obtained in the product is zero since the distance between the two nuclei is so great that the overlap of the orbitals vanishes. Sketch the boundary surface of a d x 2 −y 2 and a p y orbital.
(Bottom row) The boundary surface (left) and the cross section (right) of 3p z orbital. The boundary surface diagram of an orbital is independent of principle quantum number. There are really no boundaries defined for orbitals, in the sensecof demarcating a volume within which the electron will always be found.
In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived regions of space with different probabilities of containing an electron. (a) (LaO-AlO 2-7) free, (b) (AlO 2-AlO 2-6.5) free, (c) (LaO-LaO-6.5) free, and (d) (LaO-LaO-6.5) free with the removal of one electron. A 3s orbital is even larger, and it has three nodes.
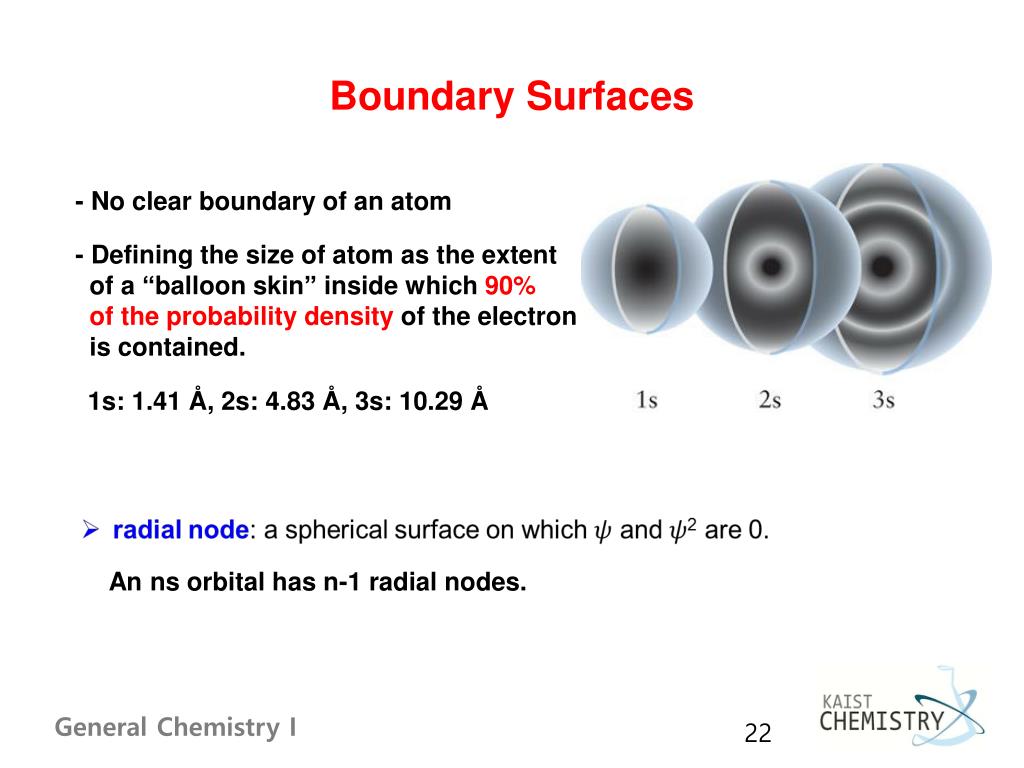
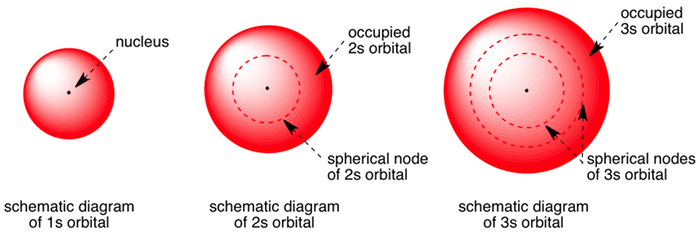
The total DOS for free standing LAO slabs under different boundary conditions. Boundary surface diagram for s orbital looks like a sphere having the nucleus as its center which in two dimensions can be seen as a circle.Hence, we can say that s-orbitals are spherically symmetric having the probability of finding the electron at a given distance equal in all the directions. Angular nodes can be visualized on boundary surface diagrams as planar nodes that separate the different signs of the orbital.
Sketch the boundary surface of a dx2−y2 and a py orbital. The orbitals in an atom are organized into different layers or electron shells. Consider the orbitals shown here in outline.
Orbitals With The Following Boundary-surface Representations. Be sure to show and label the axes. The electron is more likely to be found somewhere inside the spherical boundary surface than outside it.
This shapes encloses the volume or region where probability of finding electron is high Shape of S-orbital. For the s orbitals, these contour representations are merely spheres. Chemistry students encountering atomic orbitals for the first time often wonder why the orbital looks so different from the others.
The energy of the La 5s orbital is set as reference zero, and the arrows indicate the Fermi level. As n increases, the size of the orbital increases. The choice of the atomic orbitals needed to describe the molecular orbitals is known as the minimal basis set.
The electron is more likely to be inside this boundary shape rather than outside the boundary). At a density boundary within the ocean. A p orbital is rather like 2 identical balloons tied together at the nucleus.
According to the Heisenberg principle of uncertainty, the position and velocity of electrons in an orbital are uncertain. The d orbitals have ℓ = 2 and occur for the first time with n = 3. Boundary Surface Diagrams It is surface in the space where probability density is constant for a given orbital.
Each d-orbital has two nodal planes or angular nodes. 53 views View 1 Upvoter. L (the orbital angular quantum number) and m l (the magnetic quantum number).
Show transcribed image text. This Demonstration shows the basic characteristics for a chosen set of 16 atomic orbitals:. We call this surface a node or a nodal surface.
Hence, the shape of boundary surface diagrams of orbitals gives the shape of orbitals. Also in general terms, determines an orbital's shape, and its orientation. Be sure to show and label the axes.
The first two terms denote that one electron is on atom A and one on atom B, both with 1s atomic density distributions. Notice how the dot density diagram reveals a feature about the 2 s orbital that boundary surface does not:. All p orbitals are double-lobed, with a region of high electron density on each side of the nucleus.
Aono a The Institute of Physical and Chemical Research (RIKEN), 2-1 Hirosawa, Wako, Saitama 351-01, Japan b ERATO Aono Atomcraft Project, JRDC, 1-7-13 Kaga, Itabashi, Tokyo 173, Japan Received March. The Greek letter δ in their name refers to d orbitals, since the. The boundary surface for a d x 2 −y 2 orbital would look like this:.
Boundary surface diagram - the region of space that encloses about 90% of the electron density in an orbital 3. N (the principal quantum number);. Axes and labels can be displayed as an.
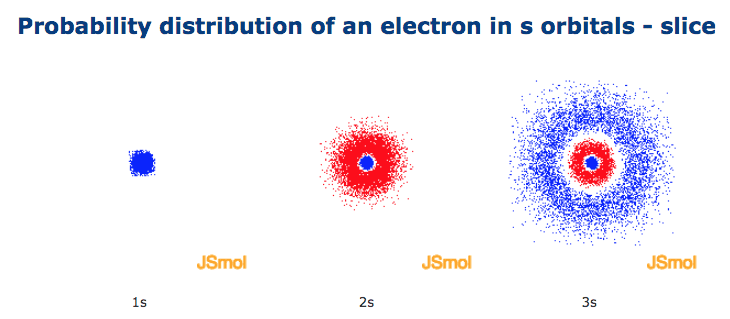
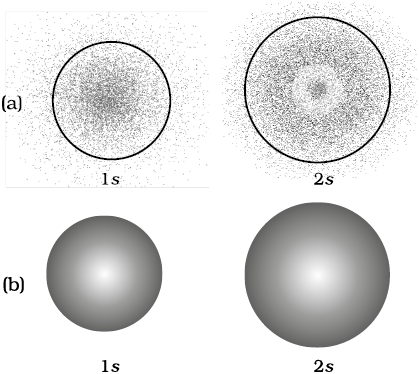
A better way of specifying how an orbital is represented is "equal probability boundary surface" which further specifies that each point on the surface must have any equal electron probablity density, and the surface must be a closed surface or set of closed surfaces with a 90% (or other percent) chance of containing the electron at a given time. 2.13 (a) Probability density plots of 1s and 2s atomic orbitals. Notice as well that the function (ls A + ls B) has the same symmetry properties as does the 1s g molecular orbital.
The type, the absolute value of quantum number , the number of lobes/nodes, the Cartesian polynomial form of the wavefunctions, and two 3D views of the probability density (boundary surface:. A node divides the 2 s orbital in two, a portion of the electron cloud is near the center, while another. Boundary surface is a representation of shape of atoms using diagram;.
Boundary Surfaces • Represent the wave function/atomic orbital in 3D – Draw a 3D surface at a given value of – Define the surfac such that it encloses a space in which the electron spends most of its time – The surface now depicts outer shape and size of the orbital – The inner structure of the wave function is hidden beneath the. The answer is related to the fact that boundary surface pictures of atomic orbitals typically show only the real part of these complex functions and often leave out the sign information as well. For example, for the 2p orbital, there is one planar node that separates the two lobes of the p orbital.
In chemistry, delta bonds (δ bonds) are covalent chemical bonds, where four lobes of one involved atomic orbital overlap four lobes of the other involved atomic orbital. There is a surface between the two balls where there is zero probability of finding an electron. The boundary surface diagrams are drawn in space for an orbital where the probability density is the maximum.
The time between two successive waves is called the _____. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). The lines in the figure represents the cross-section of the three dimensional boundary surface of p-orbitals.
Winds blowing across the ocean surface. Draw 90% probability contour (with axes) for the pz orbital. Therefore, the 2p orbital would have 1 planar node.
Shapes of f orbital. Top 3 posts • Page 1 of 1. The (Wave)functional forms for the surface which contains the 100% probability for finding the electron extends to the literal edge of the universe.
Two lobes of each p-orbital are separated by a nodal plane (a plane having zero electron density). With or without phases). Outside the boundary, the function ϕ has very small values because its square, summed over all space from the boundary wall to infi nity, has a value of only 0.1.
Other articles where Boundary surface is discussed:. One widely used method of representing orbitals is to display a boundary surface that encloses some substantial portion, say 90 percent, of the total electron density for the orbital. For s-orbital l = 0 and hence, m can have only one value, i.e., m = 0.
Each atomic orbital (Ψ) may be uniquely defined by a set of three quantum numbers:. An Orbital With An Angular Magnetic Quantum Number Of 3. This boundary surface is what is meant when the "shape" of an orbital is mentioned.
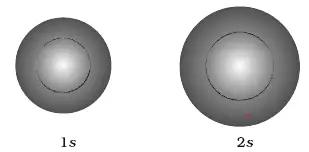
Radial probability distribution - the probability of finding an electron as a function of distance from the nucleus. Be sure to show and label the axes. A 2s orbital is similar to a 1s orbital, but it has sphere of electron density inside the outer sphere, like one tennis ball inside another.
With a hydrogen atom the probability distribution is spherical around the nucleus and it is possible to draw a spherical boundary surface, inside which there is a 95% possibility of finding the electron. 2.4.4 A look at d and f orbitals. So long as the Earth has a continental ice volume, orbital forcing will impose a 400-ky periodicity upon glacioeustasy and thereby on fourth-order sequence stratigraphy cycles.
Sketch the boundary surface of a latex{d}_{{x}^{2}\text{-}{y}^{2}}/latex and a p y orbital. For p subshells, l = 1 and has dumbbell shape. Those shapes are right, but important to note that it's not the "boundary," but rather the probability region-- where an electron is most likely to be-- if I understand correctly!.
What is the maximum number of electrons contained in an orbital of type (x)?. The three- dimensional plots of ψ^2 Vs r is clear from the above diagram of the dot- population picture or boundary surface. In this representation, a boundary surface or contour surface is drawn in space for an orbital on which the value of Fig.
Shapes of s p d or. Obviously, an orbital boundary surface defi nes an interior and an exterior. The total number of nodes of an orbital is the sum of angular and radial nodes and it is given in the terms of n and l quantum number and is given below:.
For example, for 2p x orbital. In the context of 'wave' it is nonsensical to measure an electron's position, and hence its speed may not be determined. …therefore represented by a spherical boundary surface (Figure 2), which is a surface that captures a high proportion of the electron density.
Recogniz-ing this fact allows the LCAO approximation to be interpreted in physical terms. In the helium atom there are two electrons associated with. Water waves are _____.
ELSEVIER Surface Science 344 (1995) 143-148 surface science Structure and stability of the out-of-phase boundary in a surface superlattice, Si(111)V-3 x V-3-R30Ag T. All the s -orbital are Spherical shape. Shapes of s orbital.
At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals. The five d-orbitals are designated as d xy , d yz, d xz, dx 2 -y 2 and d z2 The boundary surface diagrams of the five 3d orbitals z are shown in Fig. Previous question Next question.
Shapes of p orbital. Shapes of atomic orbitals. The sketch of 90 % boundary surface of s orbital and p x orbital is to be drawn.
The s subshell has spherical shape and does not have any sharp boundary where the chance of finding electron is zero;. The electron has a fixed energy and a fixed spatial distribution called an orbital. Shape of atomic orbitals Shape of S-orbital Boundry surface diagram Counter surface diagram Atomic structure Shape of atomic orbitals class 11th Atomic struc.
There are a total of five d orbitals and each orbital can hold two electrons. An Orbital With A Principle Quantum Number Of 1. The boundary surface means the surface which encloses 90 percent of the dots representing the electrons.
Since electron behaves as if it is spinning about an axis, a spin quantum. An atomic orbital is defined as the boundary surface encloses 95% of the electron density for a particular wavefunction Ψ. Shapes or boundary surfaces of Orbitals.
A boundary is a line or surface marking the extent of some feature, so it is not the same as an orbital. This problem has been solved!. Orbital - a three-dimensional region or shape and you can expect to find an electron inside this shape approximately 90% of the time (it may help to think of this shape as a boundary surface;.
This gives a good representation of the shape of the orbital. Therefore, the position of electrons cannot be determined with 100% accuracy. Figure 2.8 (lightbox) (Top row) The boundary surfaces and orientation of the three 2p atomic orbitals.
One way of representing electron probability distributions was illustrated previously for the 1s orbital of hydrogen. The shape of an s-orbital is spherical because the boundary. All Chemistry Practice Problems Orbital Shapes Practice Problems.
The p-orbital has 2 lobes and d-orbital has 4 lobes. The shape doesn’t depend on the principle quantum number (n). An s-orbital is spherical with the nucleus at its centre, a p-orbitals is dumbbell-shaped and four of the five d orbitals are cloverleaf shaped.
The ratio of wave height to wavelength is called the _____.
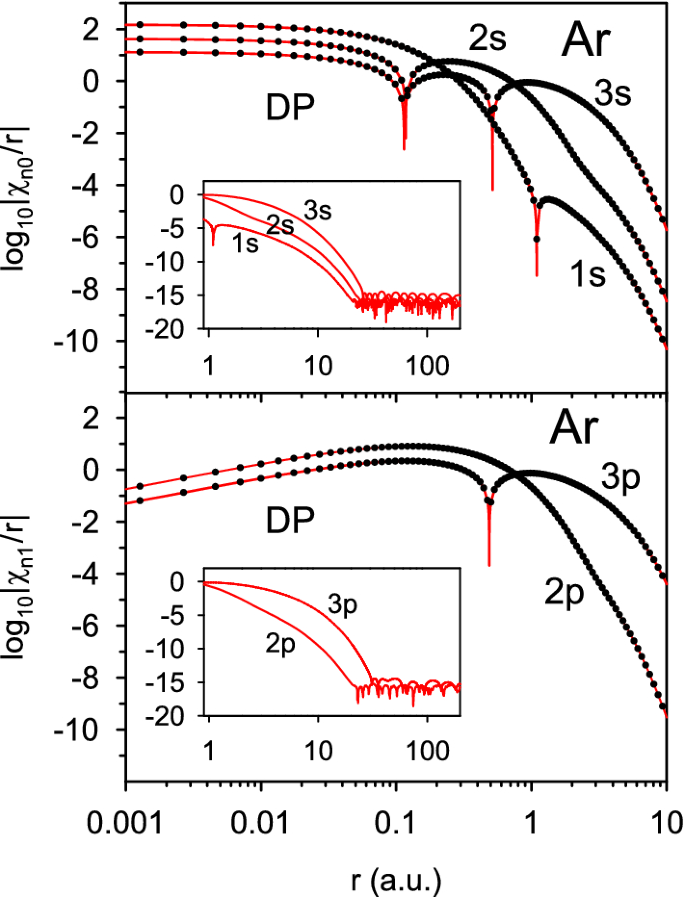
Highly Accurate Numerical Solution Of Hartree Fock Equation With Pseudospectral Method For Closed Shell Atoms Springerlink

Correlations Between States In The United Atom And Separated Atoms Limits In Heh Lee 18 Bulletin Of The Korean Chemical Society Wiley Online Library
Q Tbn 3aand9gcq5x3g0fqmejku Nwxnnh74m0xdmdqsdwowshcezmnuctzlicla Usqp Cau
Boundary Surface 5s Orbital のギャラリー
Http Eacharya Inflibnet Ac In Data Server Eacharya Documents ae36c9272b0afc9 Infiep 226 197 Et 226 197 Et V1 S1 Lec 5 Pdf

S P D F Orbitals Chemistry Socratic

6 6 3d Representation Of Orbitals Chemistry Libretexts
Http Eacharya Inflibnet Ac In Data Server Eacharya Documents ae36c9272b0afc9 Infiep 226 197 Et 226 197 Et V1 S1 Lec 5 Pdf

Metal Anions In Metal Rich Compounds And Polar Intermetallics Whangbo 11 European Journal Of Inorganic Chemistry Wiley Online Library
Quantum Theory And The Electronic Structure Of Atoms Ppt Download
S P D F Orbitals Chemistry Socratic
2
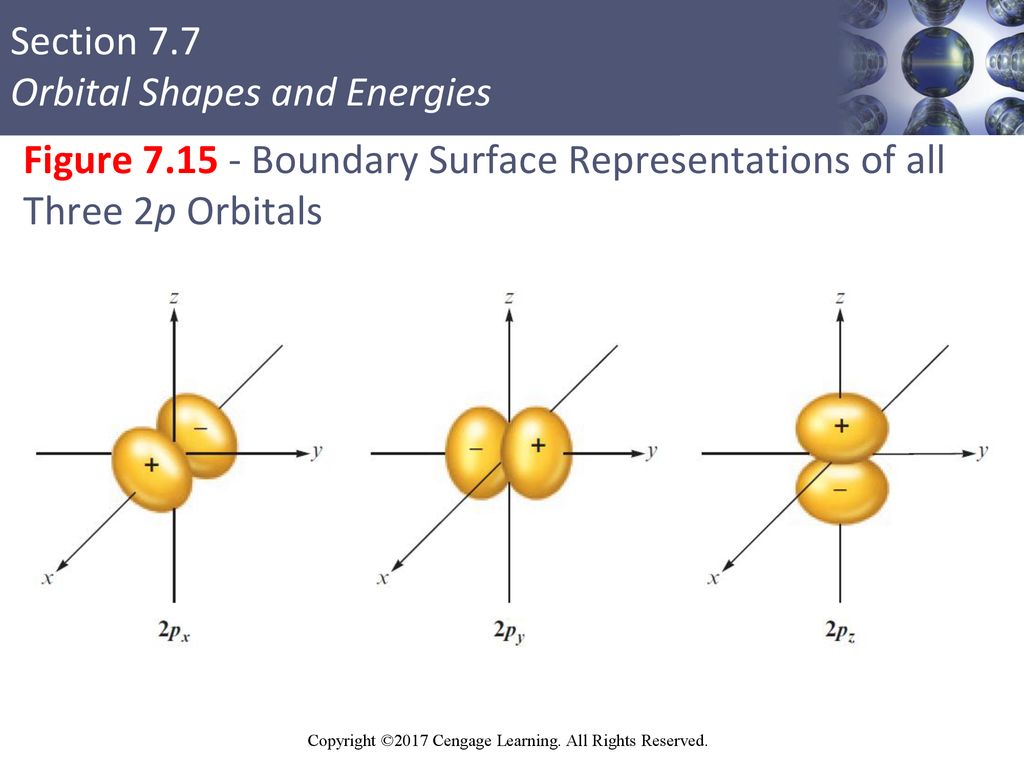
Table Of Contents 7 1 Electromagnetic Radiation Ppt Download
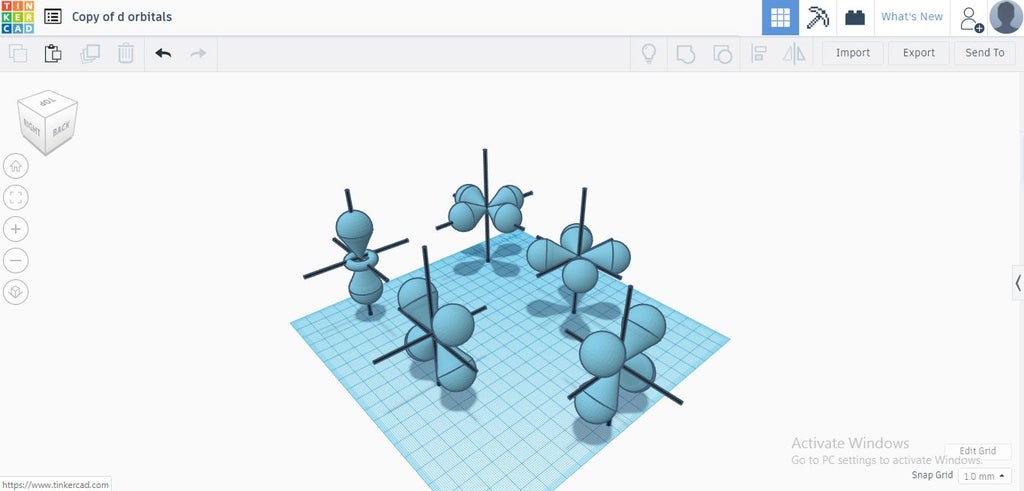
Orbitals Smoothly Explained Using Tinkercad 9 Steps Instructables
Shape Of S Orbitals In 3d

Quantum Numbers Pdf Document

Quantum Numbers Video Quantum Physics Khan Academy

Key Words Electronic Homework Problems Questions And Problems
Chemistry7 Files Wordpress Com 07 09 Lecture 30 Chapter 8 Pdf
.PNG)
Electron Densities Representations
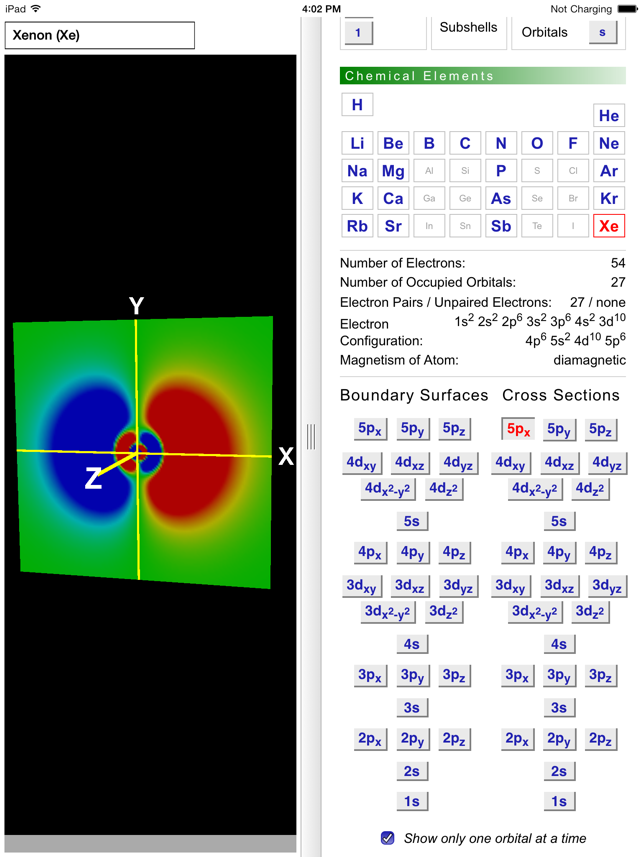
Odyssey Atomic Orbitals On The App Store
Authors Library Caltech Edu 1059 6 Tr 01 Chapter 1 Pdf

Boundary Surface Orbital Britannica
Chemistry Tcd Ie Staff People Sd Lectures Mo Lecture Course 2 Pdf

Openstax General Chemistry Ch6 Electronic Structure And Periodic Properties Of Elements Top Hat

Cover Page Chapter 7 Atomic Structure And Periodicity Ppt Download
Best Online Chemistry Notes And Study Materials Unit 2 5 Atomic Structure Shapes And Filling Of Orbitals In Atom
2
Atomic Orbitals And Their Energies
.PNG)
Electron Densities Representations
Structure Of Atom
Toward Surface Orbitronics Giant Orbital Magnetism From The Orbital Rashba Effect At The Surface Of Sp Metals Scientific Reports

Quantum Numbers And Shapes Ppt Download

Atomic Orbital Wikipedia
Stm Images A C E And Schematics B D Of Two Types Of Domain Download Scientific Diagram
Www3 Nd Edu Sevovlab Articles Jpq Pdf
Shapes Of Atomic Orbitals Boundary Surface Diagrams Of S P D Orbitals Radial Angular Nodes
Http Www Riverdell Org Cms Lib05 Nj Centricity Domain 98 Quantum numbers tutorial and practice Pdf

Shapes Of Atomic Orbitals Definition Examples Diagrams
Structure Of Atom Ncert Class 11 Chemistry
Q Tbn 3aand9gcskivjbnush9k65b8lm Ya0meyxhkn7qjkblwfh arasj8cxs Usqp Cau
Chemistry Tcd Ie Staff People Sd Lectures Mo Lecture Course 2 Pdf

Ln Boundary Surface Diagram Of Five 3d Orbitals Which Orbital Has Only Two Lobes Brainly In

Shapes Of Orbitlas And Electronic Configuration Class 11 Chemistry
.PNG)
Electron Densities Representations
2

Match Column I With Column Ii Column Icolumn Ii1 The Orbital With 4 Total Nodesa 3s 2 The Orbital With One Nodal Planeb 5s 3 The Orbital With 2
Best Online Chemistry Notes And Study Materials Unit 2 5 Atomic Structure Shapes And Filling Of Orbitals In Atom
Q Tbn 3aand9gcsepj7nlycvl8r4hqaxqj4 6tgko5qgffm6kqlvdoc6lw1anzw3 Usqp Cau

Counting Nodal Surfaces In Molecular Orbitals Elimination Of Artificial Nodes Sciencedirect
Strong And Fragile Topological Dirac Semimetals With Higher Order Fermi Arcs Nature Communications

Electron Orbitals Pogil
Electronic Structure Of Atoms And Molecules Springerlink
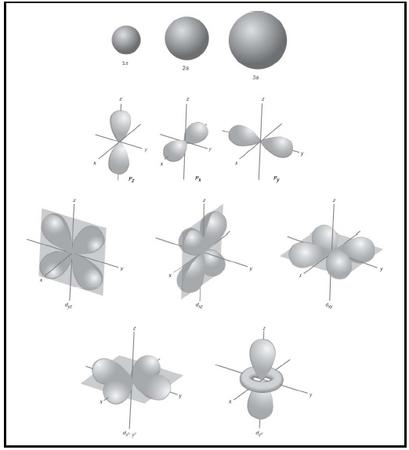
Atomic Structure Chemistry Encyclopedia Elements Metal Gas Number Name Symbol Equation Salt

Chemistry The Central Science Chapter 6 Section 6
Www3 Nd Edu Sevovlab Articles Jpq Pdf

5 8 Orbitals Chemistry Libretexts
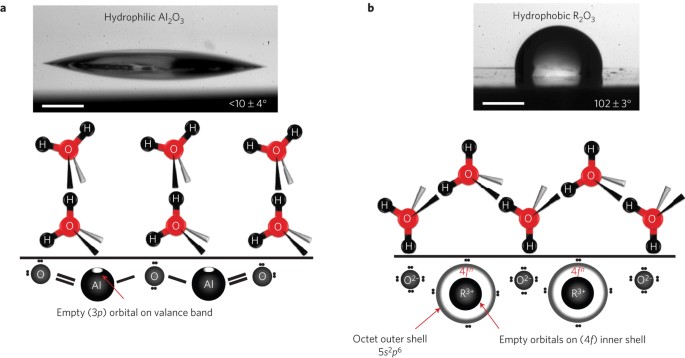
Hydrophobicity Of Rare Earth Oxide Ceramics Nature Materials

Counting Nodal Surfaces In Molecular Orbitals Elimination Of Artificial Nodes Sciencedirect
Best Online Chemistry Notes And Study Materials Unit 2 5 Atomic Structure Shapes And Filling Of Orbitals In Atom

Table Of Contents 7 1 Electromagnetic Radiation Ppt Download

Fermi Surface Plots For The 4h Polytype Of Silver A Top Download Scientific Diagram

Boundary Surface Diagram Features Constant Probability Density
2
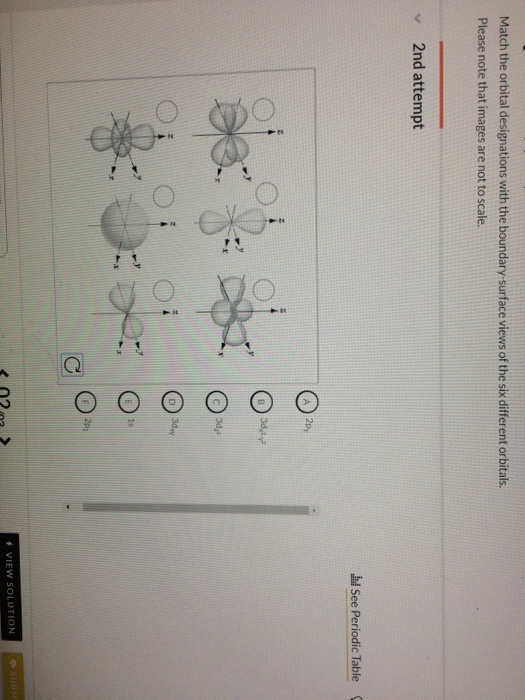
Solved Match The Orbital Designations With The Boundary S Chegg Com

Chemistry The Central Science Chapter 6 Section 6

Illustration Of The Orbital Interactions That Lead To Lone Pair Download Scientific Diagram
Ppt Quantum Mechanics And Atomic Structure Powerpoint Presentation Free Download Id
Ocw Mit Edu Courses Electrical Engineering And Computer Science 6 007 Electromagnetic Energy From Motors To Lasers Spring 11 Lecture Notes Mit6 007s11 Lec44 Pdf
Shape Of S Orbitals In 3d
2

Tips Electron Configuration With Video How Do You Get The Electron Configuration Of An Element
Shapes Of Atomic Orbitals Boundary Surface Diagrams Of S P D Orbitals Radial Angular Nodes

Atomic Orbital Wikipedia
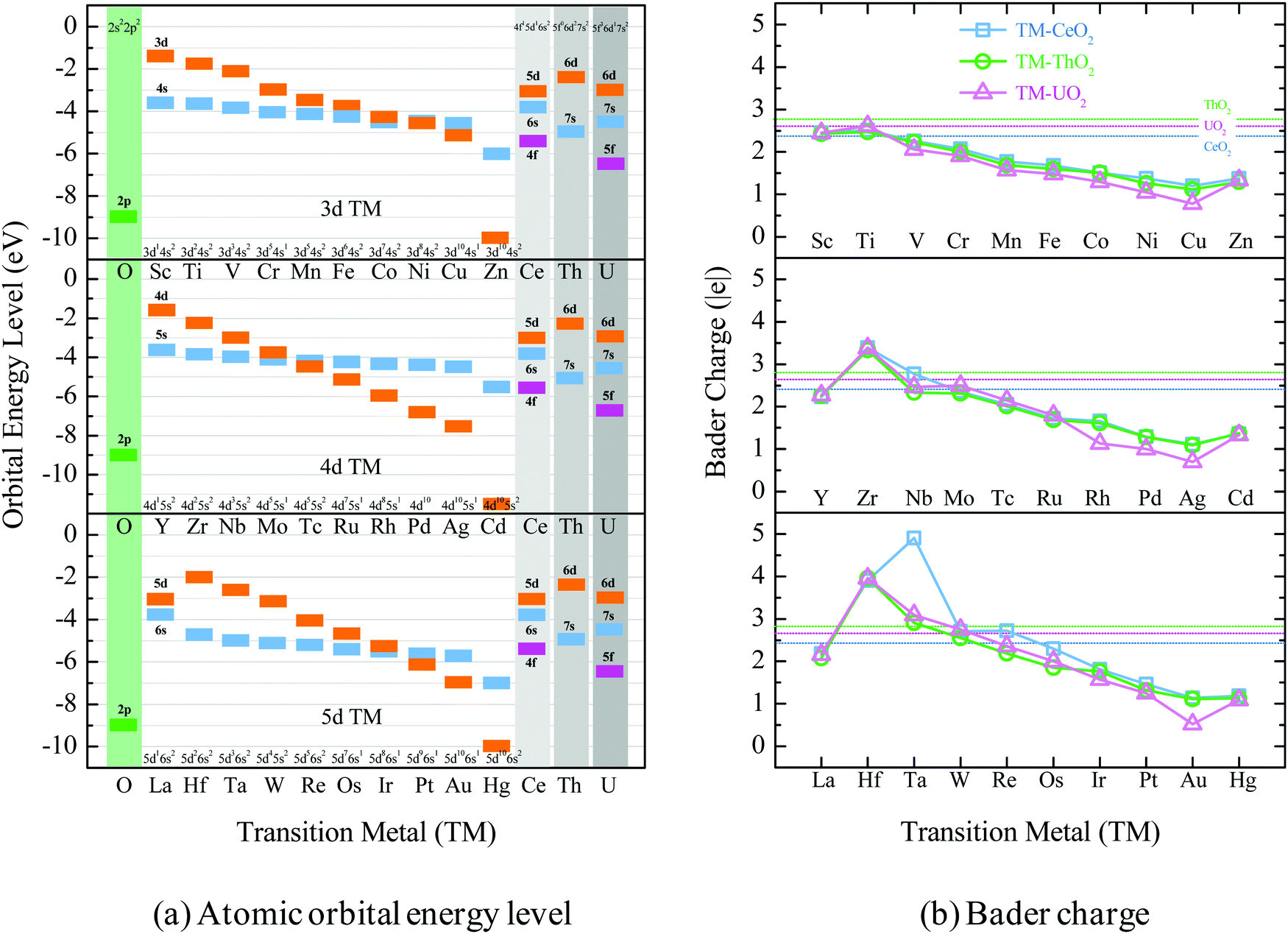
Dependency Of F States In Fluorite Type Xo2 X Ce Th U On The Stability And Electronic State Of Doped Transition Metals Physical Chemistry Chemical Physics Rsc Publishing

Atomic Orbital Wikipedia
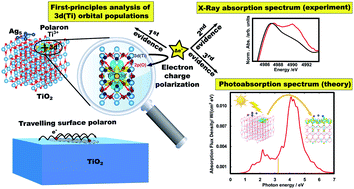
Exploring The Properties Of Ag5 Tio2 Interfaces Stable Surface Polaron Formation Uv Vis Optical Response And Co2 Photoactivation Journal Of Materials Chemistry A Rsc Publishing

Boundary Surface Orbital Britannica
2
Www Unf Edu Michael Lufaso Chem3610 Inorganic Chapter1 Pdf
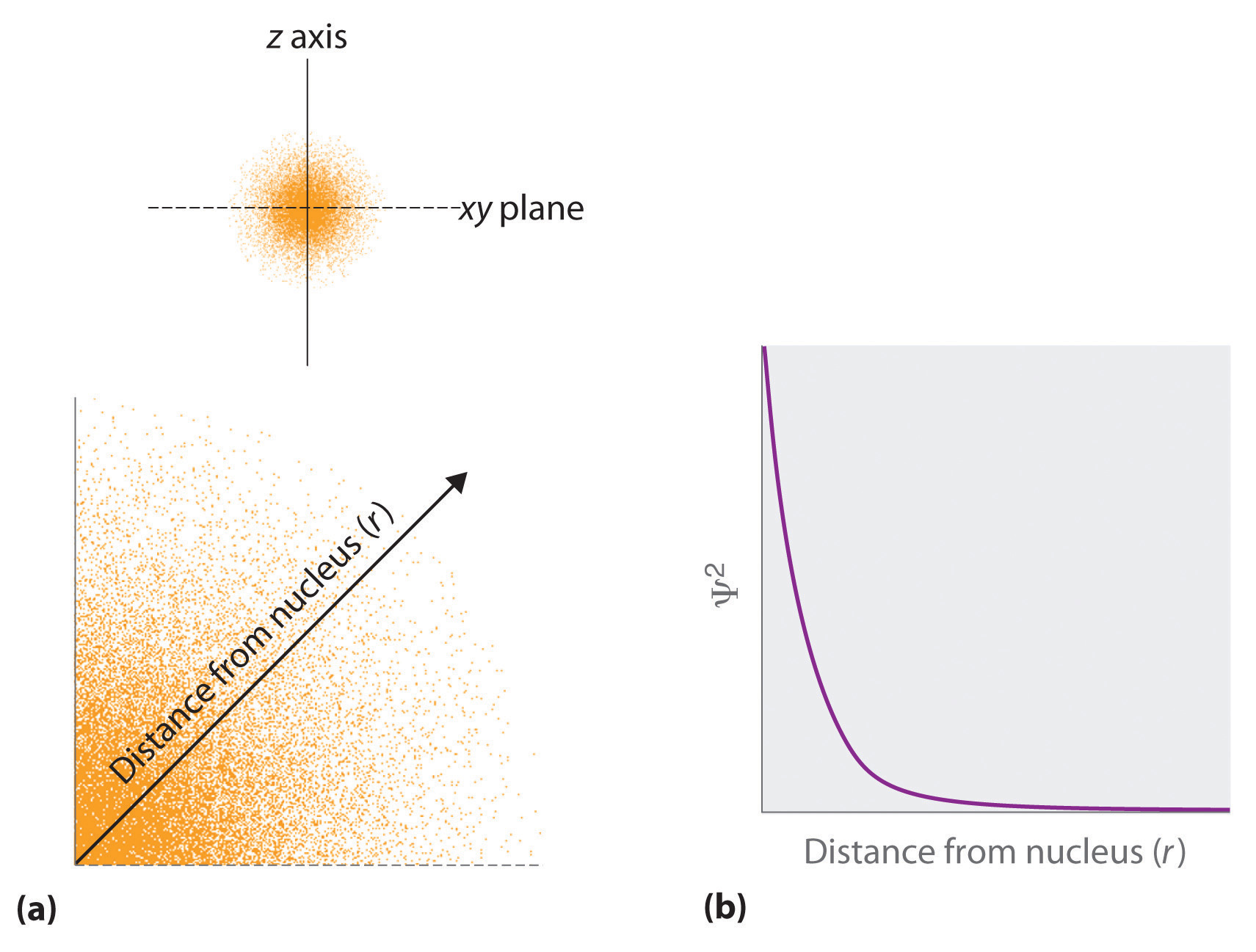
Atomic Orbitals And Their Energies
.PNG)
Heisenberg Uncertainty Principle

Surface Thermodynamic Stability Electronic And Magnetic Properties In Various 001 Surfaces Of Zr2cosn Heusler Alloy Sciencedirect

Shapes Of Orbitlas And Electronic Configuration Class 11 Chemistry
2
Www Unf Edu Michael Lufaso Chem3610 Inorganic Chapter1 Pdf

Drawing Orbitals Nodes Youtube
Authors Library Caltech Edu 1059 6 Tr 01 Chapter 1 Pdf
2

2 6 Quantum Mechanical Model Of Atom Ncert Class 11 Chemistry Books For Blind And Visually Impaired Students Blind2visionary Org

Shapes Of Orbitlas And Electronic Configuration Class 11 Chemistry
2
2

Bingorbitals
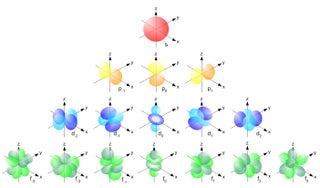
Orbitals Smoothly Explained Using Tinkercad 9 Steps Instructables
Bingorbitals

High School Chemistry Posts Facebook

Shapes Of Atomic Orbitals Part 1 S Orbital Chemistry For Class 11 In Hindi Youtube
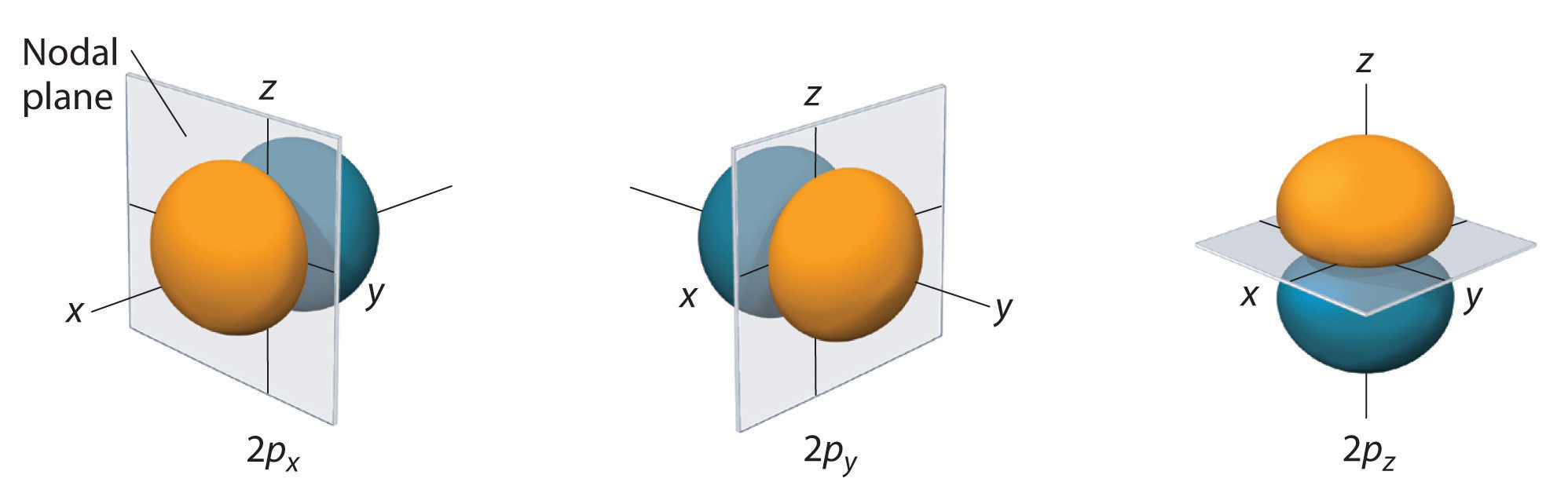
6 6 3d Representation Of Orbitals Chemistry Libretexts
Chemistry7 Files Wordpress Com 07 09 Lecture 30 Chapter 8 Pdf
Q Tbn 3aand9gcqvxh3mldsbghuwq Yaxzntmxtj9avpvx8ethjffy1sn2hs4klu Usqp Cau
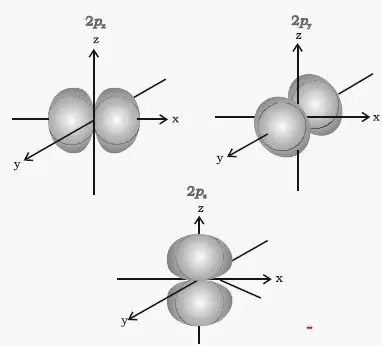
Shapes Of Atomic Orbitals Boundary Surface Diagrams Of S P D Orbitals Radial Angular Nodes
Orbitals Smoothly Explained Using Tinkercad 9 Steps Instructables

Boundary Surface Orbital Britannica

Chemistry 11 Chapter 2 Shape Of Orbitals Boundary Surface Diagrams Of S P D Orbitals Nodes Youtube




